

The number before the element name is the atomic number and that after the element name is the mass number. The mass number of uranium-238 declines by four and its atomic number by two when it emits an alpha particle. For instance uranium-238 decays into thorium-234 with a half-life of almost 4.5 billion years by emitting an alpha particle:ĩ2-uranium-238 => 90-thorium-234 + alpha particle (nucleus of 2-helium-4) Many heavy nuclei emit an energetic alpha particle when they decay. Photons of gamma rays can damage living cells by splitting molecules apart or ionizing elements in them. Photon energy is directly proportional to the frequency of the electromagnetic radiation. Gamma rays are like light, except that they are much higher frequency electromagnetic rays. It should be noted that the emission of gamma rays does not change the mass number or atomic number of the nucleus - that is, unlike radioactive decay by emission of particles, spontaneous fission, or electron capture, it does not cause the transmutation of the nucleus into another element.Įach quantum, or unit, of a gamma ray (or other electromagnetic energy) is called a photon. Gamma rays are essentially like X-rays and are the most penetrating form of radiation. Some of this residual energy after radioactive decay can be emitted in the form of high-frequency electromagnetic radiation, called gamma rays. Often, there is still excess residual energy in the nucleus after the emission of a particle or after electron capture.

But it is the most damaging form of radiation when deposited inside the body.
FISSION ENERGY SKIN
It is stopped by the dead layer of skin and so does no harm when outside the body. This is the least penetrating form of radiation. Alpha decay, which the emission of a helium-4 nucleus containing two protons and two neutrons.There are several ways in which unstable nuclei undergo radioactive decay: All isotopes of heavy elements with mass numbers greater than 206 and atomic numbers greater than 83 are radioactive. These nuclei are radioactive, in that they emit energy and particles, collectively called “radiation.” All elements have at least some isotopes that are radioactive. The nuclei of some elements are not stable. The term derives from the tendency to periodicity of chemical properties deriving from arrangements of electrons in atoms. Elements are arranged in ascending order of atomic number in an arrangement called the periodic table. The number of protons in the nucleus, which determines the chemical properties of an element, is called the atomic number. Hence the nominal mass, based on the mass number, approximates the actual atomic mass.
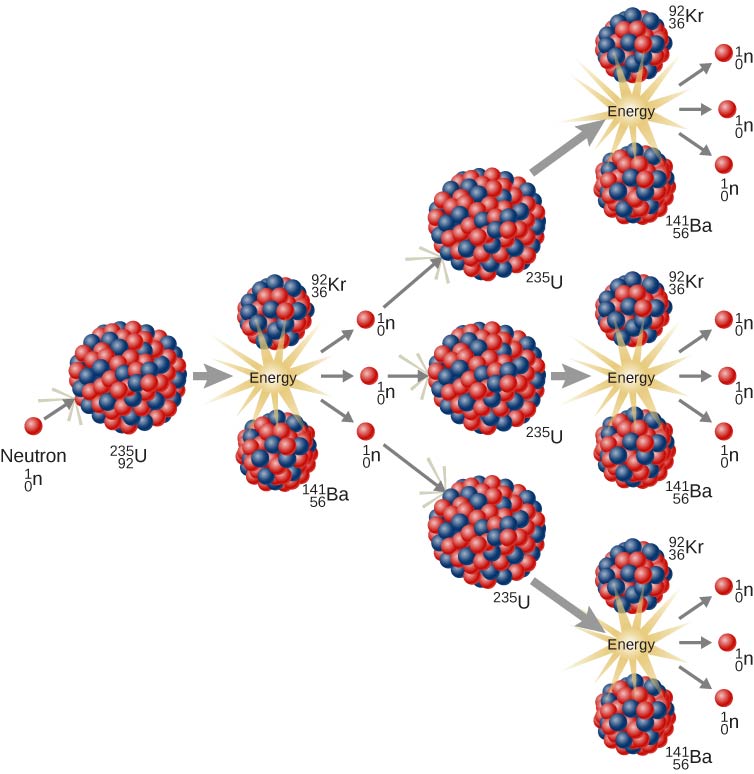
The nominal mass of an atom is not affected by the number of electrons, which are very light. The nominal mass of an atom of an element is measured by the sum of the protons and neutrons in it. The energy balance in the decay of a neutron is achieved by theĪnti-neutrino, a neutral particle that carries off surplus energy as the
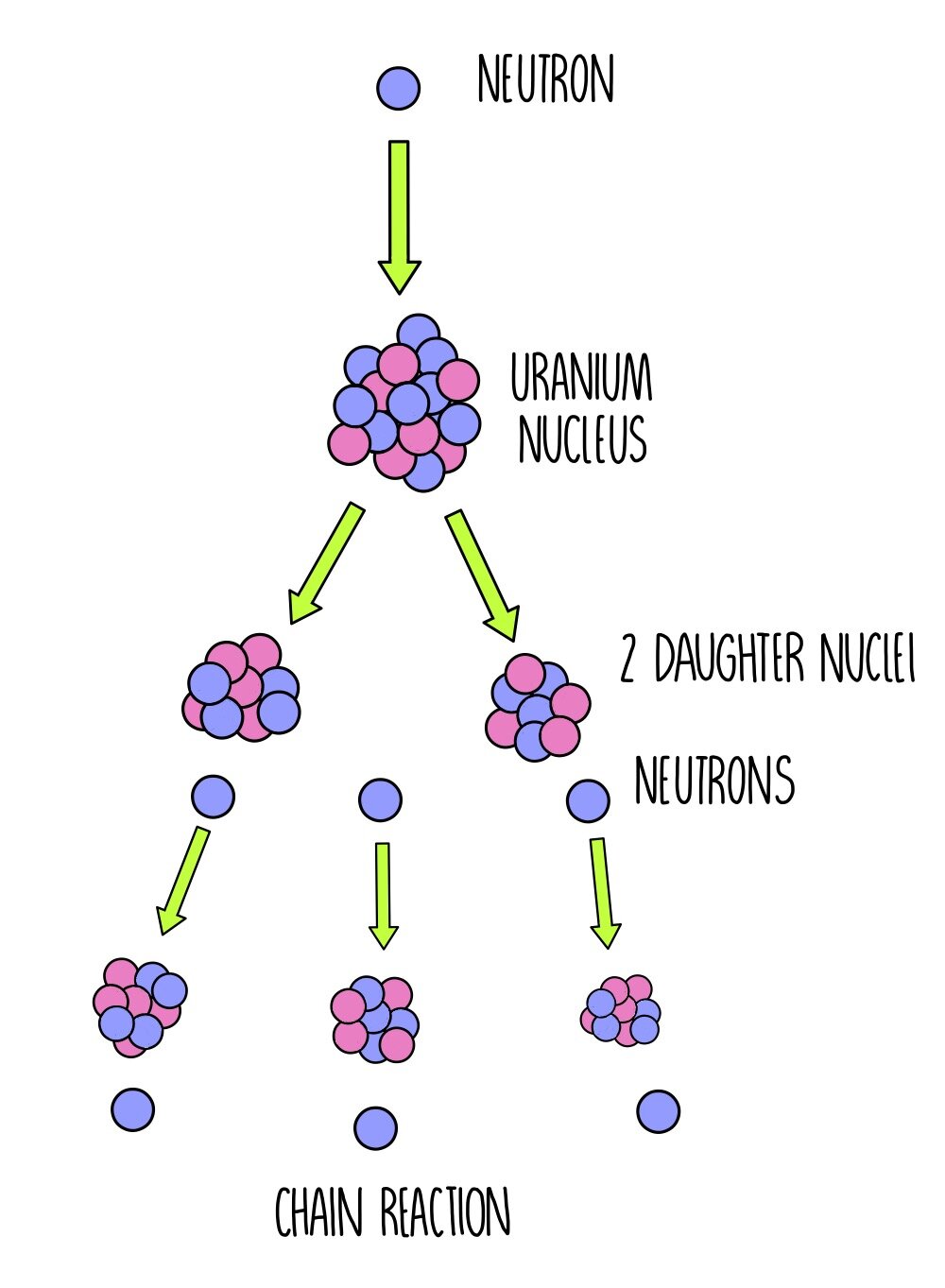
Protons are about 1,836 times heavier than electrons, and neutrons are about 1,838 times heavier than electrons. However, it is remarkable that neutrons, when they exist together with protons in the nucleus of atoms, are stable. Neutron => proton + electron + antineutrino The mass of an atom lies almost entirely in its nucleus since protons and neutrons are far heavier than electrons.įree neutrons are unstable particles which decay naturally into a proton and electron, with a half-life of about 12 minutes. As a result, atoms of elements are normally electrically neutral. The number of electrons in an atom is normally equal to the number of protons in the nucleus. Electrons are electrically negative and have a charge equal in magnitude to that of a proton. The nuclei of atoms are composed of protons, which have a positive electrical charge, and neutrons, which are electrically neutral. The atoms of which every element of matter is composed have a nucleus at the center and electrons whirling about this nucleus that can be visualized as planets circling around a sun, though it is impossible to locate them precisely within the atom. Some of the terms used in this factsheet can be found in IEER’s on-line glossary. A basic background in nuclear physics for those who want to start at the beginning.


 0 kommentar(er)
0 kommentar(er)
